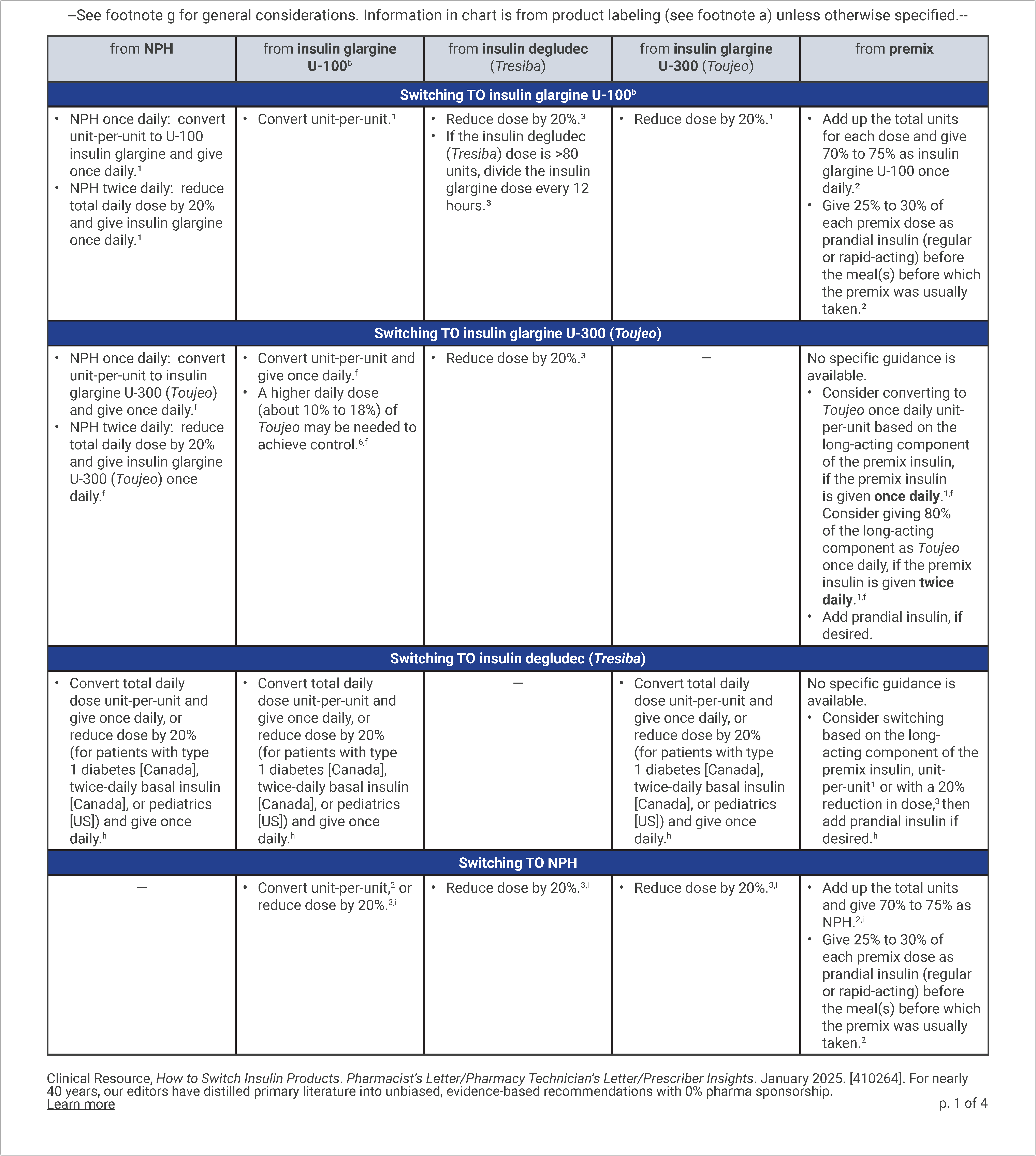
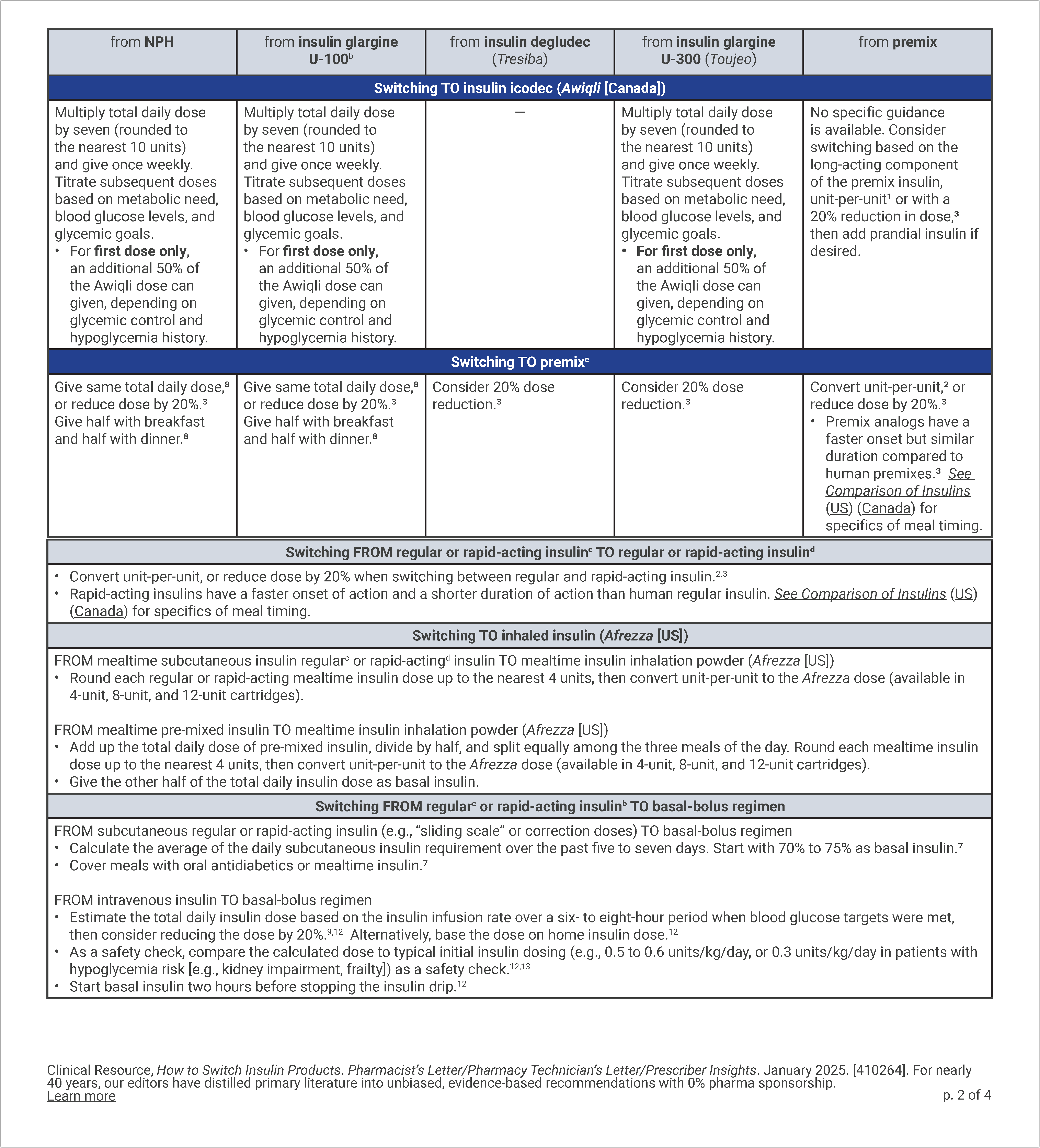
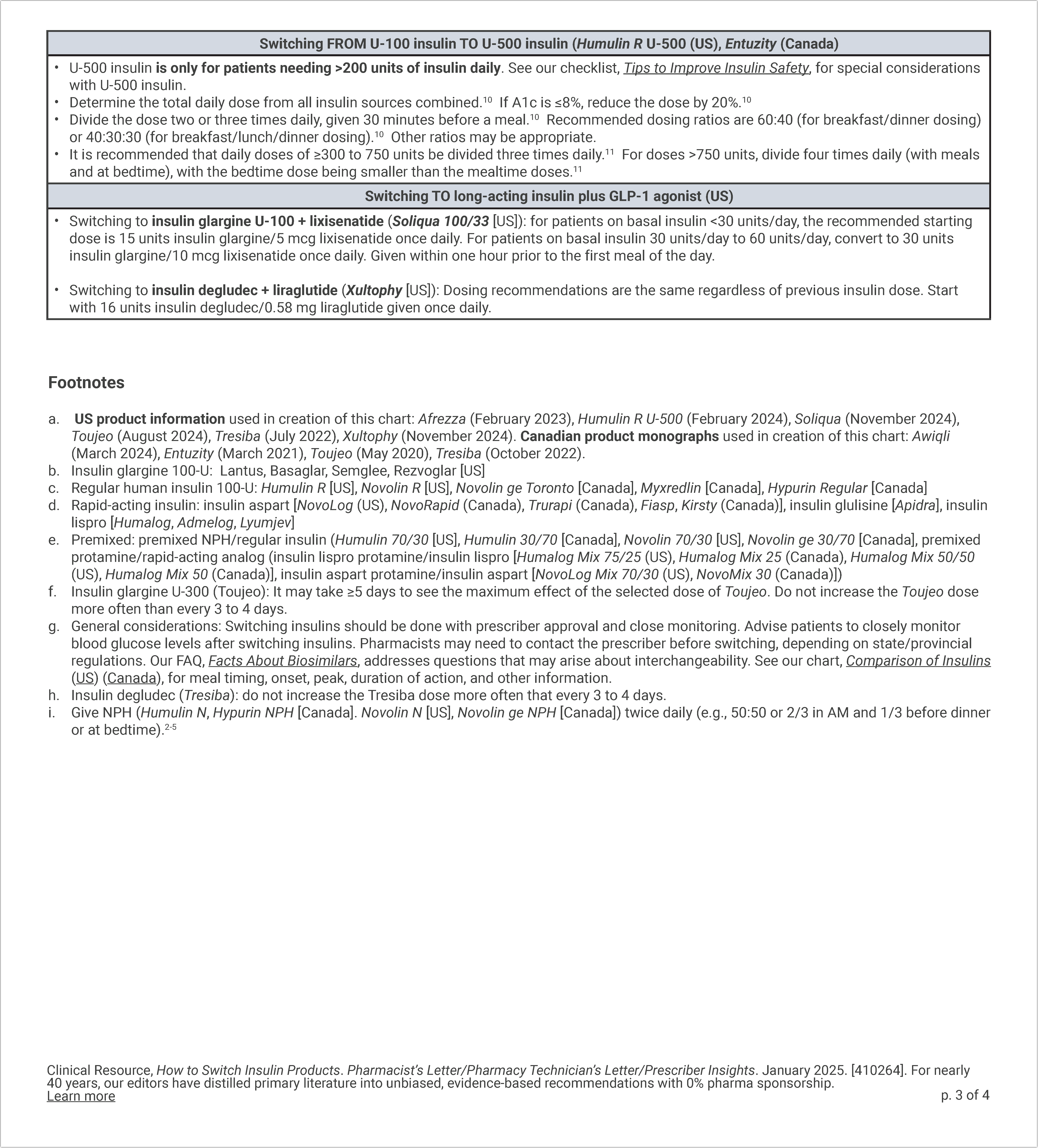
How to Switch Insulin Products
Related Articles
- Be Ready to Help Patients Switch Insulin
- Compare Lyumjev and Semglee to Other Insulins
- Help Patients With Skyrocketing Insulin Costs
- Consider Lower-Cost Insulin Regimens
- Think of Basaglar as a New BRAND of Insulin Glargine...NOT a Generic for Lantus
- Don't Expect Tresiba to Have Much Benefit Over Other Longer-Acting Insulins
- Consider NPH and Regular Insulin INSTEAD of Insulin Analogs
- Think of Toujeo as a More Concentrated Form of Lantus
- Consider Adding Insulin if Patients Are Already on 3 Diabetes Meds
- questions about how to switch between insulins